A2K Scientific Direct Amp Influenza AB Kit (RUO)- 5000 RXNS
$35,000.00
Kit Contents
- Influenza AB RT-PCR multiplex assay
- DirectAmp Mastermix
- DirectAmp Enhancer
5000 reactions per kit
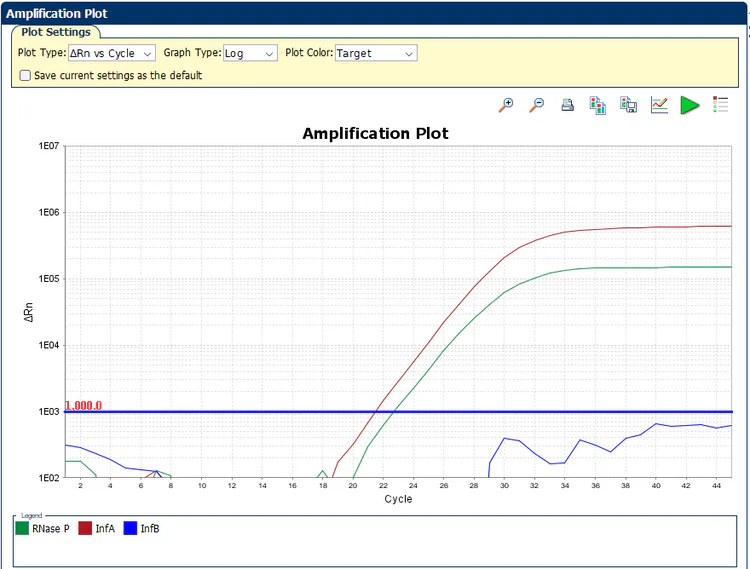
Targeted up to 100% of currently available complete genomes for Influenza A & B
Primers and probes selected from an evolutionarily well conserved regions of the M1 & NS2 genes
Targeted RNAse P gene for internal control and collection efficacy monitor
Identical in sequence to the CDC Influenza SARS-CoV-2 EUA
Technical Specifications*
-Module 1
Influenza A
Influenza B
-A2K Evaluation
Analytical Controls
Whole genome standards
Clinical Nasopharyngeal Swabs
Crude or Purified samples
-Multiplex Channels
FAM
Specifications
| Weight | 15 lbs |
|---|---|
| Dimensions | 12 × 10 × 16 in |
| Product Number | DA-FLU-5000 |
| Size | 5000 RXNS |
| Brand | A2K Scientific |
The A2K Influenza A/B Multiplex Assay is a real-time reverse-transcription polymerase chain reaction (rRT-PCR) laboratory test that can simultaneously detect and differentiate between influenza A and influenza B in upper or lower respiratory specimens. Combine the RT-PCR assay with the powerful A2K Direct amplification mastermix for reliable detection of Influenza in crude sample – No extraction required.